Viewpoint: The emerging barcode initiative for medicines in India - part 3
Avi Chaudhuri, PhD, 18-Oct-2022
Part 3 — From concept to reality: operationalizing a cost-effective programme to defeat counterfeiters and bring value to industry
The emerging barcode programme for domestic medicines represents a turning point for India in its battle against counterfeit variants. After several years of planning, the Ministry of Health and Family Welfare (MoHFW) has now issued a set of draft guidelines on how the pharmaceutical industry should proceed. Those guidelines were analysed in the first two articles of this series and recommendations made on how the programme should be revised so as to cohere with global standards, conform to best practices, and capitalize on the unique market conditions of India [1, 2].
The benefit to both government and the citizenry is immense if a national anti-counterfeiting programme is well constructed and then successfully deployed. Often overlooked in the creation of such regulatory mandates is the interest of the third pivotal stakeholder — the pharmaceutical industry. The assumption is that it will simply be a compliant participant because the laws shall compel them to do so. I reject that coercive approach to governance and instead believe that industry’s enthusiastic acceptance is essential to both engagement and sustenance.
Here, I provide a blueprint for the value proposition to the Indian pharmaceutical industry and explain why it could stand to benefit from the emerging MoHFW programme. Value is an economic derivative that is directly proportional to benefit and inversely related to cost. The key to reducing cost is to operationalize the programme in the most streamlined manner possible and if applied in conjunction with a provocative possibility, could actually end up neutralizing the financial impact. The other part of the value equation, benefit, is where things get really interesting.
Key takeaways from Parts 1 and 2
The first article in this series explored why the MoHFW regulation, as presently drafted, would not have a consequential impact on reducing drug counterfeiting in India, and could actually worsen the current situation. The static content of the proposed barcode* on a given medicine can be easily replicated by anyone with intent, thereby creating the ultimate nightmare scenario — providing false reassurance to helpless consumers that the fake medicine they happened to purchase was actually genuine.
To reduce that possibility, I suggested that each package barcode contain a unique serial number. Although it could be surmised that the agency intended to launch a serialization program, the draft regulation does not contain explicit language in this regard and therefore industry is left with a regulatory mandate that lacks clarity. A strong case was additionally made that India join other nations in ensuring that barcode content and format conform to recognized international standards to allow proper decipherability at the later reading stage by an empowered stakeholder.
Although the recommendations made in the first article would create a solid foundation, the success of any anti-counterfeiting programme requires a holistic solution design based on a well-constructed barcode. The second article presented just such a programme architecture by describing the various participants and the interactive engagements among them. While there can be various models for any given programme, the proposed design made a strong case for only pursuing an authentication protocol, rather than Track & Trace, with design elements best suited to the unique environment and complex market factors present in India.
The first two articles were largely conceptual in nature, with arguments offered on the best way forward for India. That now brings us to address the reality of making a national anti-counterfeiting programme operationally viable, against which there are the usual two obstacles — financial impact and industry acceptance. The best-laid plans can get derailed in the absence of cost containment. India is a rising economic power, but it is also a fact that a large segment of the populace faces daily struggles in meeting life’s costs. Adding a further financial burden to that reality can be indefensible. And yet, the duty of protecting society against fake medicines makes for an equally compelling counter-argument, as now recognized by the Indian government. Next, I present an implementation plan that balances the opposing demands of these two business and societal forces.
A roadmap for operational success
A well-designed mass serialization programme can offer substantial protection. However, placing a unique number on a drug package is not a trivial task, compounded by the further challenge of ensuring seamless authentication outcomes. To ensure success, both drug companies and the MoHFW must be uncompromising in pledging that every saleable drug package covered under the mandate contains a readable barcoded, without exception or error.
Direct marking versus label application
Placing a serialized barcode on each drug package can be undertaken in two ways — direct online coding or application of a coded label. Online coding is not new to the Indian pharmaceutical industry, where the practice has been undertaken for a few years now for exported products. The cost factors here include procurement of high-resolution printers and vision systems to validate the print matter, both operationally integrated with online rejection systems.
Although ongoing operating cost is favourable with online coding, the economics of implementation and production impact also need to be considered. Brand owners must additionally ensure that vendor excellence is given the highest priority. Unlike India’s export serialization program, the MoHFW mandate is intended for the home market. Any deficiencies in print quality, data integration, or consumer authentication will reduce programme confidence and sully that brand owner’s public reputation. Companies taking the online coding approach should therefore only recruit well-established solution providers with known technology prowess, a track record of implementation excellence, and commitment to ongoing support.
The second approach to serialization is to apply a barcoded label on the package. The deployment method here can involve online label printing and application. There are several excellent vendors that provide such print-and-apply machinery. A significant advantage to this approach is that the drug package is endowed with a visually salient and identifiable token for authentication. Whereas a barcode printed directly on the package is likely to become lost in the midst of all the other print matter, a security label would stand out visually and also provide the further advantage of identifying any attempts at removal due to the standard practice of procuring tamper-evident labels.
Label application would have a similar initial cost compared to online coding, though ongoing cost would certainly be higher. There is however one type of drug package for which label application represents the only viable approach. And as it turns out, a small amendment by the MoHFW to barcode content would even make it highly economical.
The challenge of coding blister packs — revisited
As argued in the first article of this series, it is essential that the MoHFW programme fully encompass blister packs, and in fact should make it a compulsory requirement for inclusion in the barcoding requirement. Otherwise, India will just not have an effective and worthy anti-counterfeiting program.
The difficulty of variable data printing on foil packages is well known but can be overcome with specialized high-cost machinery. That would just not work in India, or in any cost-sensitive market for that matter. The application of security labels in this instance would actually be the best solution because of deployment simplicity and economy. Furthermore, a small content twist in the barcode can make things especially interesting for drug companies.
Currently, the legal requirement for placing batch number and expiry date on blisters is met by an overprinting process with low-cost dot-matrix printers. The MoHFW requirement can be met by applying a small security label that would contain a barcode with just two data items (GTIN and serial number) and possible agency branding, as shown in the left panel of Figure 1. It is not necessary for the barcode to contain additional content that will in any case be directly printed online by way of the dot-matrix printers.
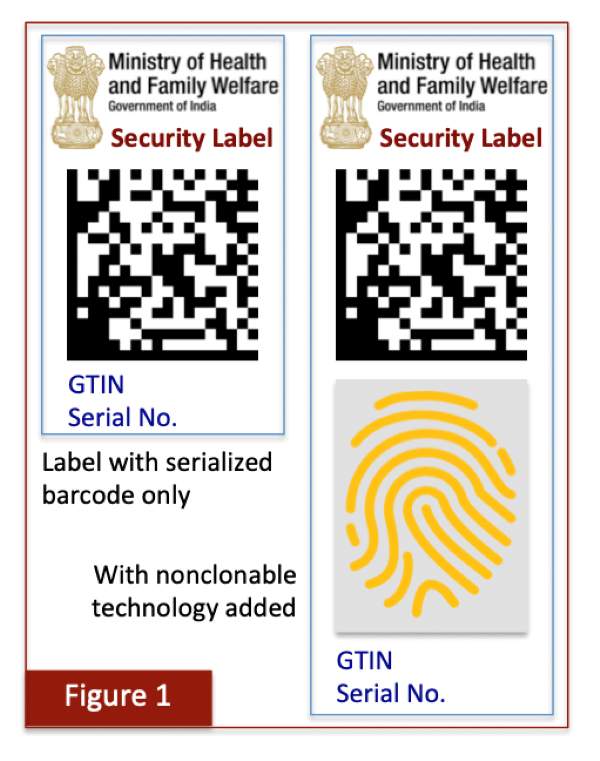
Label application on blister packs would offer several key advantages. The first is that brand owners can procure pre-printed rolls of barcoded labels in advance from a reputable supplier. This is because the data contents in the barcode are not dependent on production because both GTINs and serial numbers are pre-determined. Second, any requirements to associate batch details or other data proposed by the MoHFW, as spelled out in the draft regulations, can be undertaken during production by electronic means in the backend without having to deploy an expensive online printing setup. The associated data would then be instantly accessible during authentication. Brand owners would need to recruit a worthy solution provider to work in conjunction with the label supplier and ensure that printed barcodes are correctly associated with MoHFW-mandated data on the packaging line, and then certify proper commissioning for later authentication in the market.
The third advantage is the substantial savings in capital expenditure because there is no need to procure expensive print-and-apply systems. All that is needed is an applicator on each packaging line because only pre-printed labels have to be affixed. The only other machinery requirement is a standard barcode reader to commission (activate) the coded packages going to market.
It has been proposed in the previous article of this series that medicines that are especially sensitive or vulnerable to counterfeiting should require an additional protective nonclonable feature, such as a fingerprinted token. The purpose of this additive is to guarantee that any copied labels will be instantly flagged as being suspect so that not even a single authentication event would yield a false outcome. A label supplier with access to such technology can embed and deliver this additional security layer on the pre-printed labels when needed, as shown in the right panel of Figure 1.
Taken together, a pre-printed label application scheme would provide all of the solution requirements for India, with the additional advantage of offering the most cost-effective approach to blister coding. That discussion is taken up next.
Cost containment strategies
The MoHFW programme proposed in this series of articles has focused on two primary outcomes — maximizing success and minimizing cost. The suggested amendments to the draft regulations are meant to be as parsimonious as possible by limiting content overreach and creating a highly streamlined operating structure. Only the most essential requirements for a successful anti-counterfeiting campaign have been incorporated in the solution design.
One example of the streamlined approach is to remove, at least for now, the temptation to undertake a Track & Trace program. As explained in the second article of this series, the vast size and diversified nature of the Indian drug supply chain would make that an untenable task. There is also just no need to propose such a cumbersome programme that would in any case fail to curtail the presence of fake drugs because of the many ways the electronic supply chain can by bypassed.
An important outcome of avoiding Track & Trace is cost reduction. Item-level traceability is a highly involved process, both at production and across the entire supply chain. Rather, an authentication-based programme would be far simpler to implement and at a fraction of the cost, and most importantly, will provide far greater protection to Indian consumers.
Cost estimation
It is always a tricky process to estimate programme costs because of the many factors involved, the two most important being the volume under consideration at a particular site and which of the two coding methodologies is deployed. Online coding would require capital expenditure at the outset to retrofit packaging lines with needed printers and vision inspection equipment. The cost of this undertaking would vary depending on type of product being coded (e.g., bottle, carton, etc.), production throughput, available IT resources, and so on. The earlier concern that the cost could exceed one million dollars per packaging line is however highly overstated [3].
The second coding approach, application of pre-printed labels, was discussed above for blisters, though a similar tactic can also be undertaken for other package types, notably bottles and cartons. The cost advantage of the labeling approach is that it would greatly reduce machinery investment on each packaging line because only an applicator is needed. And in some cases, manual application can also be pursued, as widely practiced in India. The only other initial cost would be related to programme setup and customization, which again would depend on site details as above.
As for ongoing cost, preliminary estimates place it around 0.5 INR (USD 0.006) per security label, inclusive of printing, application, data migration and authentication services. This cost could even be somewhat less with large orders and the fact that the non-proprietary nature of serialized labeling would invite healthy competition among solution providers with a verifiable track record of excellence.
The NPPA factor
The final aspect of cost is a proposal that is both provocative and yet sensible, and which would have a truly affirmative effect on the MoHFW programme. The Indian government set up a federal agency in 1997, known as the National Pharmaceutical Pricing Authority (NPPA), to ensure availability of certain drugs and implement price controls on essential medicines [4]. Any increment in cost of these drugs must first be approved by the NPPA before being passed on to the market.
The NPPA has allowed price increases in the past to accommodate the extra cost of deploying an anti-counterfeiting solution. An early effort at controlling counterfeits was to embed a holographic strip on genuine packages, for which the NPPA allowed drug makers to increase their price by 0.08 INR [5]. The effort was largely unsuccessful because holograms were known to be ineffective in curbing counterfeits due to the ease with which they can be duplicated to near perfection [6]. NPPA then allowed a far larger price increase in 2012 when drug prices could be hiked by as much as 1.02 INR to accommodate a proprietary nonclonable anti-counterfeiting technology [7].
It is clear that the NPPA has established precedents for allowing reasonable price increments so that drug makers do not have to alone absorb the cost of undertaking product security. As discussed above, the proposed MoHFW programme can be accommodated via coded security labels at an estimated cost of 0.5 INR, a price point that is entirely reasonable with the streamlined model proposed in the second article of this series, and which roughly falls at the midpoint of NPPA’s prior two price accommodations.
It would therefore be reasonable for the NPPA to permit a price increment now to ensure that a comprehensive and effective MoHFW programme can be implemented. While there would certainly be setup costs for industry, an NPPA allowance to recover running costs up to 0.5 INR per item would be a fair proposition to protect Indian consumers. The pharmaceutical industry could lobby for such an allowance because it would be a fair way to meet the twin objectives of cost containment and protecting society, with the least possible impact to all stakeholders.
Benefit enhancement strategies
A persistent thread in this article is that a strong value proposition to industry would make it a willing and engaged partner in the MoHFW programme. One way to find the sweet spot between ensuring protection and accepting protection is through cost containment. That objective can be achieved through the suggested programme structure described above, in coordination with allowance of a modest price increase by the NPPA.
Next, I take up the benefit component of the value proposition to drug companies, where the investment will not only reduce counterfeiting to protect their customers, but also offer remarkable new opportunities in growing business through consumer engagement.
Engagement-driven brand protection
Let's first ask a simple question — how many companies are prepared to ask their customers to verify that a purchased drug of theirs is actually genuine? The question is theoretical because no formal studies have been carried out, though the answer can surely be surmised to be very low. Brand owners rarely disclose that their products are under attack. And this in turn sets up a dilemma for the MoHFW — a protective programme no matter how well constructed is doomed to failure because of consumer apathy arising from either passive ignorance or poor incentivization.
One way to circumvent this obstacle is to motivate consumers to interact with the product because in return they will derive something of value. Fortunately, there are numerous options for arousing customers when it comes to medicines, as shown in Figure 2. All of these efforts are aimed at satisfying a basic human desire — the yearning for information and to be in the know on all matters concerning their personal health. And that in turn forms a powerful driver for patients to engage with drug companies because they will derive a direct and personal benefit.
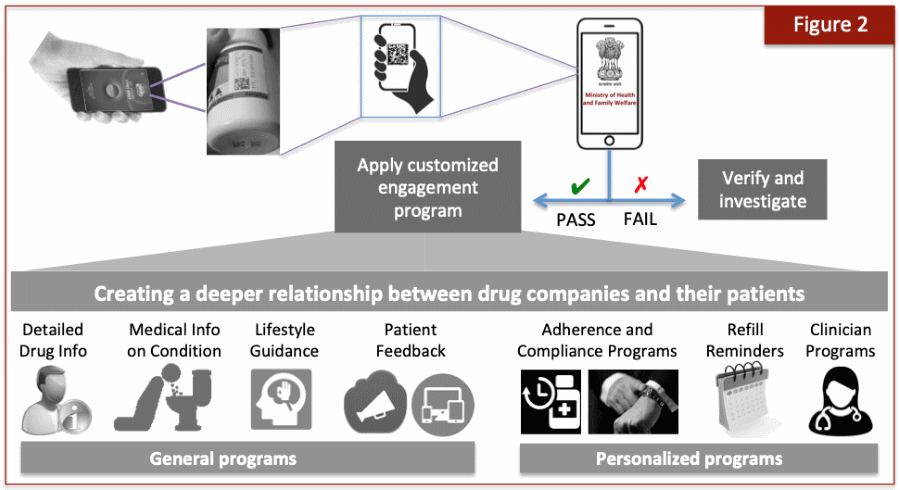
Connected packaging offers multiple engagement possibilities
The mere act of serialization can transform a ‘dumb’ drug package into one with tremendous possibilities for interactive engagement and communication with patients, a trend that has been dubbed connected packaging [8]. The engagement possibilities can be placed into two categories. As Figure 2 shows, a set of general programmes can be delivered that provide content value, such as details on the drug itself and information on the medical condition, both in digestible form to ordinary laypersons.
The drug company can also provide lifestyle guidance for those drugs used to treat chronic conditions, such as diabetes, hypertension, etc. This can be an invaluable service, delivered right to the mobile phone without the need to undertake further search efforts. And in return, the company can obtain direct feedback from patients, such as positive outcomes as well as any adverse side effects that may be experienced.
In addition to the general programmes, several options are available for personalized engagement customized to the specific needs of the patient. There are a large number of possibilities but a particularly valuable one is to ensure patient adherence to the drug. It has been found that patients with chronic conditions have an adherence level of only around 50%, whether in North American or Asian populations [9, 10]. This remarkable result means that only about one-half of patients on long-term therapeutics actually adhere to their prescribed regimen.
The health care impacts from such non-adherence are immense and therefore considerable effort is being made to tackle the problem [11]. The advent of package serialization can add to that palette of options by providing alarms, adherence tracking, and reminder notification, including refills. A new digital era of adherence tracking can be introduced at the patient level because each medicine pack will have its own digital identity.
Finally, clinician-based programmes offer novel options to receive technical details, research publications, adverse drug interactions, side effect management, and much more. Together, the availability of general and personalized engagement to the brand owner is truly striking. The more creative brand owners will be able to unleash impressive interactive programmes that will be welcomed by their patients.
Connected packaging offers business benefits to brand owners
The MoHFW programme opens up fascinating possibilities for drug companies to participate in interactive mobile engagement. The digital world has rapidly changed and eyeballs are now on the mobile device as the gateway for that very engagement. The business of selling drugs can be rewarded through creative value offerings to patients. Brands that offer opportunities to create a deeper relationship with their customers will have substantially greater appeal to both the prescribing doctors and paying patients. And in turn, those companies will be rewarded with business intelligence, patient loyalty, brand growth, and increased revenue.
The benefit component of the value proposition therefore provides a striking opportunity to enlist industry in the anti-counterfeiting campaign and encourage it to become an eager partner. The MoHFW should not only allow but actually encourage brand owners to offer consumer programs. The greater the offerings in the marketplace, the greater the overall benefits to society.
The final requirement for success
In closing this article it is important to re-emphasize the critical importance for attaining the widest possible consumer acceptance, and with it, a strong and willful reception by the public to undertake product authentication. Consumer acceptance will unfold automatically if the government can make the case that it has created an excellent and reliable platform for protection, with rapid verification outcomes that provide on-the-spot assurance at the time of purchase. Many of the design elements outlined in these articles have focused on creating a programme with exactly that purpose.
The key challenge for both government and industry is to maximize drug authentications once the programme has been launched. A lack of public adoption will be a crying shame and a missed opportunity after much effort has gone into launching a national program. It is therefore critically important to undertake extensive efforts at public awareness and motivate the highest level of authentication. That must be a shared objective of the agency, drug companies, and national media.
A concerted effort at public education will undoubtedly have substantial success. Consumer authentications have a multiplicative impact due to the vast number of inspections across the marketplace. And that in turn would serve not only to detect counterfeit drugs but also deter their appearance. Criminals do not wish to be caught. The greater the number of inspections undertaken by the general public, the less the likelihood of criminal activity due to the possibility of immediate detection and interdiction. And that outcome will represent an enduring testament to success.
Synthesis
The publication of the MoHFW draft regulation has spurred sudden hope toward fulfilling the national desire to eliminate counterfeit drugs across India. One unfortunate outcome, however, is the appearance of press reports that the draft regulation in present form will be an instant panacea, and that the counterfeiting problem will just simply go away [12]. That will not happen. If the application of a mere static barcode on a package could eliminate counterfeit drugs, then such a simplified approach would have been deployed long ago across all markets.
The barcode is instead merely the foundation for supporting a holistic programme whose design must be customized to the unique needs of a particular market. The trio of articles in this series has offered just such a solution for India. The overall design and its various intrinsic components were based on learnings from the global marketplace and best practices borne of both success and failure in prior attempts.
The following is a brief summary of six key elements offered in this trilogy for the Indian programme:
- The barcode to be placed on drug packages should contain only a limited set of data, follow international standards in terms of structure and content as developed by GS1, and embed a unique serial number. Each drug package is therefore individually serialized.
- All saleable packages, including notably blisters, must be included in the programme and is therefore subjected to serialized barcoding. Those medicines deemed to be sensitive or especially vulnerable to counterfeiting should contain an additional nonclonable technology, such as a fingerprinted token.
- The MoHFW programme should only follow an authentication requirement and avoid implementing Track & Trace. Furthermore, consumers must be empowered as authenticating agents through use of a single mobile application offered by the government that will serve as the unitary gateway for all drug verifications.
- The mobile application would in the first instance direct all traffic to the MoHFW (or other appointed agency) for drug identification by way of the GTIN, after which the product owner would undertake serial number verification and notify the consumer accordingly.
- An ideal way to operationalize the programme across all package types, and blisters in particular, is to apply pre-serialized labels. This approach can be undertaken in quick order and at reasonable cost. The NPPA should allow drug companies to recover up to 0.5 INR per item, which would be sufficient to cover the operating cost of a label-based solution.
- The MoHFW should permit and even encourage the drug industry to capitalize on the barcoding programme to introduce consumer engagement programmes that create a deeper relationship with customers and usher greater public knowledge about disease, therapy, and drug adherence.
The MoHFW has been very wise to initiate a programme with just the top 300 drugs. This cautious approach would help to ensure that any deployment obstacles are limited and can be quickly resolved. The foundational elements of an effective programme have already been proposed in the MoHFW draft. The amendments and operational revisions offered in this trio of articles would allow India to have one of the most successful anti-counterfeiting programmes anywhere, and one that fully meets the distinctive needs of this market in an effective, economical, and enduring manner.
References
[4] https://journalsofindia.com/silver-jubilee-of-national-pharmaceutical-pricing-authority-nppa/
[6] https://www.securingindustry.com/pharmaceuticals/the-case-against-holograms/s40/a9353/#.YzhiCS1h3zI
[9] https://www.uspharmacist.com/article/medication-adherence-the-elephant-in-the-room
[10] https://pubmed.ncbi.nlm.nih.gov/33515135/
[11] https://patientengagementhit.com/news/3-patient-centered-steps-to-reduce-medication-non-adherence
* The use of the generic term barcode throughout this article refers only to the two-dimensional barcode that has been proposed by the MoHFW, and not the linear barcode used for retail scanning found on all packages.

Dr Avi Chaudhuri is founder of The Kulinda Consortium, a global alliance of solution providers that focuses on emerging nations to protect their citizens from counterfeiting, illicit trade, and revenue loss. His work in this field began when he became the victim of a counterfeit drug while traveling in India nearly two decades ago.
Dr Chaudhuri introduced the very concept of serialization to the Indian pharmaceutical industry in 2007, which later set in motion India’s drug export serialization programme. He is an acclaimed expert in the field of anti-counterfeiting, working with both governments and the private sector.
Top photo by Naveed Ahmed on Unsplash
Related articles:
- Maiden Pharma has registration nixed over Gambia deaths
- DEG contaminant identified in cough syrups linked to Gambia deaths
- Viewpoint: The emerging barcode initiative for medicines in India - part 2
- Gambia bans paracetamol syrup after child deaths
- Viewpoint: The emerging barcode initiative for medicines in India - part 1
- Boehringer, SAP blockchain app authenticates medicines
- Global meds regulators publish track and trace recommendations
- Evotaq joins with Movilitas to deliver drug traceability to UAE
- Pakistan’s MedznMore makes tackling fake meds its mission
- Bahrain presses go on its track and trace plan for medicines
- Blockchain medicine tracking pilot gets nod in Afghanistan

Click here to subscribe to our newsletter
18-Oct-2022
Related articles:
- Maiden Pharma has registration nixed over Gambia deaths
- DEG contaminant identified in cough syrups linked to Gambia deaths
- Viewpoint: The emerging barcode initiative for medicines in India - part 2
- Gambia bans paracetamol syrup after child deaths
- Viewpoint: The emerging barcode initiative for medicines in India - part 1
- Boehringer, SAP blockchain app authenticates medicines
- Global meds regulators publish track and trace recommendations
- Evotaq joins with Movilitas to deliver drug traceability to UAE
- Pakistan’s MedznMore makes tackling fake meds its mission
- Bahrain presses go on its track and trace plan for medicines
- Blockchain medicine tracking pilot gets nod in Afghanistan
Sponsored Content
The Dark Side of Imitation – Counterfeit Alcohol and the Fight for Safety
The Transported Asset Protection Association (TAPA) has published a white paper on the scourge of counterfeit alcohol in response to a string of high-profile incidents that have claimed hundreds of lives.
The document details some of the most notorious incidents in recent years and explains how a brand protection standard (BPS) developed by TAPA Asia Pacific (TAPA APAC) can strengthen the supply chain against counterfeit goods by encouraging logistics companies to "design out" vulnerabilities. Read more
Sponsored Content
The Dark Side of Imitation – Counterfeit Alcohol and the Fight for Safety
The Transported Asset Protection Association (TAPA) has published a white paper on the scourge of counterfeit alcohol in response to a string of high-profile incidents that have claimed hundreds of lives.
The document details some of the most notorious incidents in recent years and explains how a brand protection standard (BPS) developed by TAPA Asia Pacific (TAPA APAC) can strengthen the supply chain against counterfeit goods by encouraging logistics companies to "design out" vulnerabilities. Read more
Press Releases
-
》 Energous introduces battery-free e-Sense tag, establishing first end-to-end wireless power platform for the ambient IoT
-
》 TrusTrace launches 4th industry playbook: A new framework to streamline data collection to comply with industry regulations and de-risk supply chains
-
》 Crane Authentication unveils InsightPulse: A discreet, smartphone-enabled authentication system
-
》 Antares Vision Group partners with Siempharma to enter pharma labeling equipment market with automatic unit for cylindrical containers
Partners



