Milestone looms for NanoGuardian as first product nears market
Phil Taylor, 07-May-2010
 NanoGuardian is anticipating the launch of the first commercial pharmaceutical product to make use of its brand protection technology within the next few months, marking a major milestone for the two-year-old group.
NanoGuardian is anticipating the launch of the first commercial pharmaceutical product to make use of its brand protection technology within the next few months, marking a major milestone for the two-year-old group.
The launch of the product from a top five pharmaceutical manufacturer will provide another endorsement of the firm's NanoEncryption technology, which can be used to protect individual tablets, capsules and other dosage forms from counterfeiting and diversion, according to Dean Hart, executive vice president of NanoGuardian which operates as a division of parent company NanoInk.
He told SecuringPharma.com that the first commercial product launch will mark the culmination of a multi-year collaboration with the unnamed drugmaker, which was instrumental in testing the NanoEncryptor device used to add the security features to pharmaceutical products.
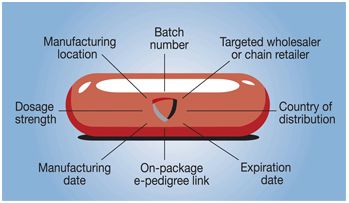
In simple terms, NanoEncryption involves 'punching' encrypted overt, covert and forensic marker patterns onto the surface of a dosage form without the use of any inks or other chemicals.
The overt feature can be identified with the naked eye, while the covert feature is placed within the overt mark and requires the use of a microscope or jeweller's loupe to see. The third, forensic marker - known as a NanoCode - is at the micron-scale and can only be read using specialist scanning electron microscopy equipment.
The NanoCodes can be linked to a wealth of information on the specific dose on which they are carried, including the time and place of manufacture, batch number, lot number, strength and expiry date. It can also be associated with on-pack coding to combine track-and-trace with authentication.
Back in 2008, NanoGuardian's pharmaceutical partner was planning a rapid roll-out of a different product using the NanoEncryption technology, but decided against proceeding after a patent challenge led to extensive generic competition for the blockbuster brand, according to Hart.
The company decided nevertheless to incorporate the security features into one of its pipeline products, and this is currently under regulatory review.
Meanwhile, NanoGuardian achieved another significant milestone last month when Pfizer's softgel capsule division Capsugel took out an exclusive license to the technology, allowing it to provide pre-coded capsule shells to its customers.
Capsugel is the leading manufacturer of gelatin capsules with a global market share estimated to be in excess of 50 per cent, ahead of rivals such as Shionogi Qualicaps and Cardinal Health (RP Scherer).
That deal, along with the publication of the US Food and Drug Administration's draft guidance on physico-chemical identifiers (PCIDs) such as chemical taggants last year, provides evidence that the pharmaceutical industry is starting to embrace the concept of on-dosage security features, according to Hart, and that is good news for NanoGuardian.
"By the end of next year I believe we could see five to 10 different products making use of the NanoEncryption technology," he predicted.
In anticipation of that increased demand, NanoGuardian is also planning to increase its authentication capacity, something which is currently handled at a specialist facility operated by the company in Chicago, USA.
The division plans to open additional facilities in the UK, Switzerland and Japan as use of the technology expands. Hart believes most customers will elect to have NanoGuardian carry out authentication of suspect samples, although he noted one potential customer is enquiring about the possibility of bringing the authentication equipment in-house.
Beyond oral dosage forms
Meanwhile, NanoGuardian continues to develop the technology, and recently completed a six-month programme to refine the NanoEncryption process for use in single-use syringes and vial caps, extending the application of the technology beyond oral dosage forms.
Hart said a number of requests from Big Pharma and biotechnology companies had prompted a development programme to perfect the process of adding its marker and coding technology to syringe plunger rods and vial caps.
NanoGuardian intends to supply pre-encrypted rods and caps to customers in a model analogous to the Capsugel agreement, but will handle the coding itself as the volumes are not as high as with tablets or capsules.
A pharmaceutical manufacturer will be able to use his usual bulk supplier, but the caps or plunger rods can be sent to NanoGuardian for coding and separation into the required quantities.
"This development is of particular interest for biopharmaceutical manufacturers, which are experiencing increasing problems with counterfeiting, diversion and theft," said Hart.
Related articles:
NanoGuardian joins forces with investigations firm
Capsugel deal takes NanoGuardian up a gear
NanoGuardian hires ex-FBI, pharma specialist

©
SecuringIndustry.com
 | back to top
| back to top




 NanoGuardian is anticipating the launch of the first commercial pharmaceutical product to make use of its brand protection technology within the next few months, marking a major milestone for the two-year-old group.
NanoGuardian is anticipating the launch of the first commercial pharmaceutical product to make use of its brand protection technology within the next few months, marking a major milestone for the two-year-old group.