US medical device sector gears up for unique identifiers
Phil Taylor, 20-Jul-2012
The US pharmaceutical sector may have to wait a little longer for a federally-mandated traceability system for medicines, but the situation is very different for medical devices with a final rule due by year-end.
The recently-ratified FDA Safety and Innovation Act (FDASIA) introduced a requirement for a unique device identifier (UDI) for medical devices, that was swiftly followed by the publication of a proposed rule by the FDA.
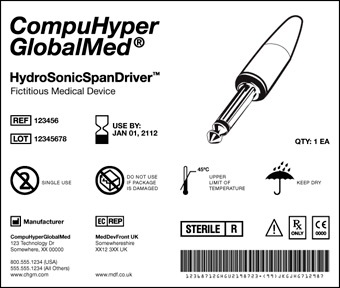 According to the agency's vision, the UDI would include a unique numeric or alphanumeric code that would identify the device (see mock-up), as well as a production identifier that would contain additional information such as the name of the manufacturer, the type of device, and a serial number, as well as the expiration date and batch/lot number.
According to the agency's vision, the UDI would include a unique numeric or alphanumeric code that would identify the device (see mock-up), as well as a production identifier that would contain additional information such as the name of the manufacturer, the type of device, and a serial number, as well as the expiration date and batch/lot number.
The information would be included in a publicly-available database, and some devices would be excluded from the requirements under exemption clauses for low-risk items. One example would be over-the-counter devices which tend to already carry a universal product code (UPC).
"A UDI system has the potential to improve the quality of information in medical device adverse events reports," said the agency, noting this will help "identify product problems more quickly, better target recalls, and improve patient safety."
The agency also points out that the system could help to address diversion, theft and counterfeiting in the medical device sector, a problem which came to the fore in 2010 when counterfeit surgical mesh products were found in the US supply chain.
There have also been major recalls of medical devices in recent years because of safety issues, including defective hip implants from Johnson & Johnson's DePuy unit and heart defibrillator leads made by Medtronic.
Publication of the proposed rule kicks off a 120-day comment period, although the agency notes it has already been in close consultation with stakeholders and the draft "builds upon current standards and systems already in use by some companies".
Coding standards organisation GS1 applauded the release of the proposed rule, with GS1 USA's vice president for healthcare, Siobhan O'Bara, saying: "a UDI system leveraged by all constituents in the US supply chain will improve the speed and accuracy of product recalls as well as adverse event reporting".
"The industry is ready, willing and able to implement UDI to the benefit of patients, healthcare providers and manufacturers here in the US and around the world," she added.
The system has been many years in gestation, given that the requirement to set up a UDI system was first introduced in 2007 as part of the Food and Drug Administration Amendments Act (FDAAA). Section 226(a) of the FDAAA noted that the UDI "shall adequately identify the device through distribution and use, and may include information on the lot or serial number".
FDASIA sets out a requirement for the US Secretary of Health and Human Services to finalise the regulations "not later than six months after the close of the comment period", which effectively sets a deadline for enactment of the system by the end of this year.
Implementation of the final rule takes place in phases over seven years, a timeframe that has attracted criticism from some quarters. The FDA has published estimates saying it would cost US companies approximately $550m in total to implement the proposal over a 10-year time frame.

©
SecuringIndustry.com




 According to the agency's vision, the UDI would include a unique numeric or alphanumeric code that would identify the device (see mock-up), as well as a production identifier that would contain additional information such as the name of the manufacturer, the type of device, and a serial number, as well as the expiration date and batch/lot number.
According to the agency's vision, the UDI would include a unique numeric or alphanumeric code that would identify the device (see mock-up), as well as a production identifier that would contain additional information such as the name of the manufacturer, the type of device, and a serial number, as well as the expiration date and batch/lot number.