 A pharmaceutical industry-backed pilot study looking at the feasibility of applying unique codes to pharmaceutical products to allow authentication at the point of dispensing has now got underway.
A pharmaceutical industry-backed pilot study looking at the feasibility of applying unique codes to pharmaceutical products to allow authentication at the point of dispensing has now got underway.
The European Federation of Pharmaceutical Industry Associations (EFPIA) is running the pilot project in pharmacies in Sweden and says it plans to pass around 100,000 products through the pilot through to its conclusion around the end of November.
The pilot is part of EFPIA’s response to the European Commission’s proposal for a mass serialisation of medicinal products, one of the measures proposed in its draft directive on counterfeit medicines.
EFPIA officially unveiled the pilot at an event in Stockholm earlier this week. At the meeting, Brian Ager, Director General of EFPIA, said: "Individual product verification will not provide a complete solution to the challenge of counterfeit medicines."
"Nevertheless, as part of a package of measures, this type of end-to end verification system will make a significant contribution to product security and reinforce patient confidence in the legitimate supply chain."
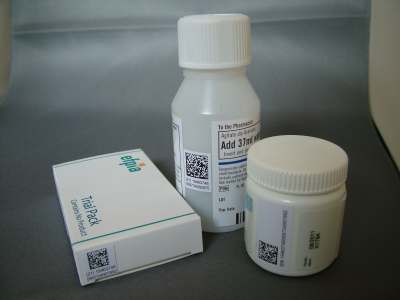
As detailed in our earlier profile of the project (see Sweden will host EFPIA serialisation project), the scheme involves marking each of the test pharmaceutical packages (pictured) with a 2D datamatrix code that will be verified at the point of dispensing in the pharmacy. As a back up to the data matrix, the randomised code, which can be up to 20 digits in length, will also appear in a human-readable text form.
 The code is based on GS1 standards and comprises the Global Trade Item Number (GTIN), a random serialisation code, expiry date and lot number, and is designed to take into account the varying coding systems across Europe.
The code is based on GS1 standards and comprises the Global Trade Item Number (GTIN), a random serialisation code, expiry date and lot number, and is designed to take into account the varying coding systems across Europe.
Scanners are used in the retail pharmacy to read the code and pass it to a verification system that checks that the pack with that serial number has not been dispensed before. If it is already marked in the database as dispensed, the pharmacist is made aware of the possibility that the pack may be counterfeit and an alert is triggered.
The pilot will involve 25 Apoteket AB pharmacies in the greater Stockholm area and local wholesalers Tamro and Oriola, with the information technology elements provided by Siemens. The wholesalers will place the data matrix codes on product packs delivered from the pharmaceutical manufacturers, said EFPIA.
The number of pharmacies enrolled into the pilot is a little lower than originally envisaged, because it is now thought that fewer will be required to meet the 100,000-product target and cutting the total number allowed the project to get underway sooner, said an EFPIA spokesman.
While other projects looking at serialisation of products in the pharmaceutical supply chain such as the EU's BRIDGE project have relied on test batches of medicines, EFPIA's pilot will handle medicines intended for eventual use by patients.
"We wanted the project to reflect the real world as far as possible," Colin Mackay, EFPIA's director of communication and partnerships, told SecuringPharma.com.
A total of 14 difference pharmaceutical manufacturers are taking part in the project, and a broad range of different products have been included to ensure that a "representative sample" of medicines goes through the system, he added.
"The products are a cross-section of various formulations, batch sizes etc," said Mackay.
That is important because EFPIA's view differs from that laid out in the EC draft directive on the scope of the measures. The draft is recommending a risk-based approach for applying anti-counterfeiting features to medicines, while EFPIA "is of the opinion the strategy should be inclusive," according to Mackay.
Selective implementation could simply drive the counterfeiters to target softer targets, he suggested.
EFPIA also wants the pilot to show the feasibility and effectiveness of an 'end-to-end' verification system, rather than the concept of 'uninterrupted traceability' which is cited in the draft directive and suggests that a full electronic pedigree approach, with verification carried out each time a medicine changes hands, may be required.
EFPIA has always argued that an e-pedigree scheme would be unworkable in an EU context given the wide range of logistical practices and software used across member states.
A comparable end-to-end verification system, developed by Aegate, is already in place in three European markets, namely Belgium, Greece, and Italy.
Even if the pilot is successful, it will still leave a number of important questions to be answered, including who will have ownership and access to the data generated in such a serialisation system, who will foot the bill for implementation, and the development of governance models that meet national regulatory and legal standards.
©
SecuringIndustry.com




 A pharmaceutical industry-backed pilot study looking at the feasibility of applying unique codes to pharmaceutical products to allow authentication at the point of dispensing has now got underway.
A pharmaceutical industry-backed pilot study looking at the feasibility of applying unique codes to pharmaceutical products to allow authentication at the point of dispensing has now got underway. The code is based on GS1 standards and comprises the Global Trade Item Number (GTIN), a random serialisation code, expiry date and lot number, and is designed to take into account the varying coding systems across Europe.
The code is based on GS1 standards and comprises the Global Trade Item Number (GTIN), a random serialisation code, expiry date and lot number, and is designed to take into account the varying coding systems across Europe.