
There is just over a week to go before drugs sold in Russia will have to have a unique, serialised code to allow them to tracked through the supply chain, but industry groups are pushing for an enforcement delay.
Russian lawmakers met a few weeks ago to hear requests from industry for some breathing space on the implementation of Russian Federal Law No. 425-FZ (decree No 1556), which states by July 1 some pharmaceutical products will need to carry the special identifiers on packaging to enable them to be tracked through the supply chain from manufacturer to end user.
Russia’s State Duma is reported to be considering a three-month enforcement holiday to make sure that all participants in the supply chain have to time to prepare. At the time of writing that had not been confirmed, but local media reports suggest that an announcement is due imminently.
Unlabelled medicines produced before July 1, can be in circulation until their expiration date, according to the legislation.
Test phase completed
In the meantime, Russia's Federal Service for Supervision of Health and Social Development (Roszdravnadzor) said this week that more than 300 organizations from 65 regions – including pharmaceutical distributors, pharmacy chains and clinics – successfully tested the movement of labelled drugs during a testing week held between June 1 and 11.
The test covered the transfer of information in the FGIS MDLP system over some basic transactions, namely acceptance, direct/reverse order, movement, withdrawal from circulation, and disposal of drugs, says the agency, which claims the test had a 99 per cent accuracy rating.
Most state-run and municipal medical organisations are already registered for the scheme, as are pharmacies, but there are still gaps to plug when it comes to private clinics, according to Roszdravnadzor.
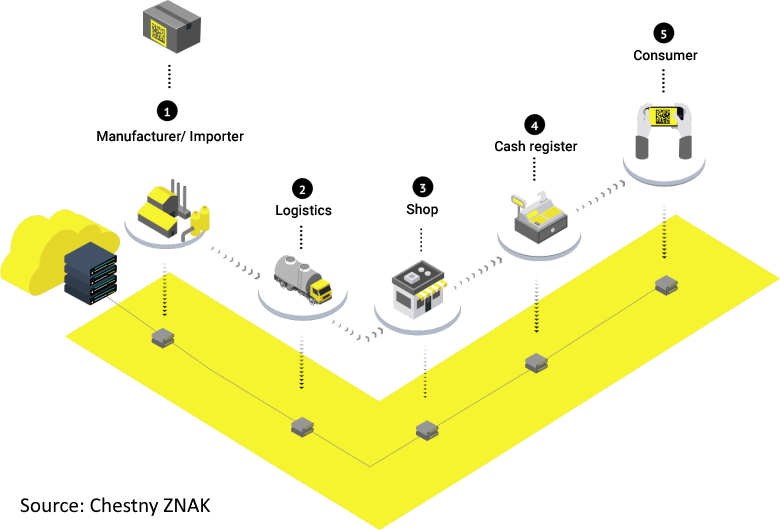
Like the EU Falsified Medicines Directive (FMD) and the Drug Supply Chain Security Act (DSCSA) in the US, Russia’s system requires a 2D barcode on all individual medicine packs, with a Global Trade Item Number (GTIN), serial number, batch number, and expiration date.
Unlike other track-and-trace systems however, Russia has also opted for a code with a cryptographic element – or ‘crypto-code’ – and a Foreign Economic Activity Common Nomenclature (FEACN) number.
The cryptographic ‘tail’ consisted of a verification key and an electronic signature, adding to the identification element which included a Global Trade Item Number (GTIN), batch number, expiration date, and serial number.
Manufacturers have to request crypto-codes from the Center for Research in Perspective Technologies (CRPT), which manages Russia’s national track and trace system, which is known as Chestny ZNAK.
The initial form of the crypto-code would have resulting in a 2D datamatrix almost double the size of what had to be printed for Europe, for example, and the Russian government partially backtracked on that plan last year by shortening the code from 88 to 44 characters.
The cryptographic element wasn’t included at all in a pilot phase started in 2017, but does allow offline authentication, giving the system an advantage over other approaches like the FMD, although it does add to the data exchange requirements.
Under the regulations, all medicines, including over-the-counter (OTC) drugs, have to carry the 2D barcode, and aggregation of all units – the individual medicine pack to the case, and the case to pallet – is also required. Supply chain partners have to report all changes within individual batches to the government.
It’s worth noting that serialisation based on this model is being rolled out across other product categories between 2019 and 2024, including tobacco and footwear – also coming under the July 1 deadline – as well as tyres, dairy products, wheelchairs, perfumes and light industrial goods in the coming months.
©
SecuringIndustry.com






