Sponsored Content
Do you have an important story about counterfeiting to tell?
Building a new brand is one of the most exciting things any business can do, but all too often that hard work will be undermined by bad actors who will try to cash in on that hard work. Sadly, most companies will experience a problem with counterfeits at some point and, for smaller firms, it can affect their ability to survive.
Here at SecuringIndustry.com, we are always ready to hear about the problems fakes pose to brand owners and are especially interested in the ways businesses rise to the challenge.
If you can share your experiences, do let us know at newsdesk@securingindustry.com.
Sponsored Content
Do you have an important story about counterfeiting to tell?
Building a new brand is one of the most exciting things any business can do, but all too often that hard work will be undermined by bad actors who will try to cash in on that hard work. Sadly, most companies will experience a problem with counterfeits at some point and, for smaller firms, it can affect their ability to survive.
Here at SecuringIndustry.com, we are always ready to hear about the problems fakes pose to brand owners and are especially interested in the ways businesses rise to the challenge.
If you can share your experiences, do let us know at newsdesk@securingindustry.com.
Press Releases
-
》 Cirba Solutions launches proprietary CirbaSuite portal, enhancing traceability and circularity across the battery supply chain
-
》 Antares Vision Group launches exception management system for pharmaceuticals supply chain
-
》 Systech selected by Praxis Packaging for track and trace solutions
-
》 Domino Printing Sciences celebrates production of 1000th R-Series machine vision inspection system with Lake Image Systems
Partners
Home | About us | Contact us | Advertise | Links | Partners | Privacy Policy | | RSS feed  | back to top
| back to top
© SecuringIndustry.com




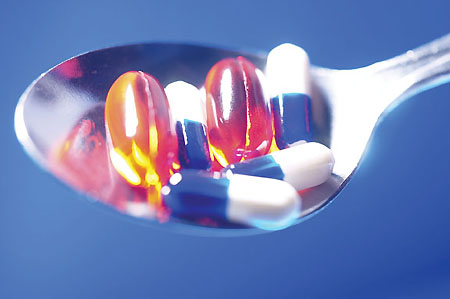 Companies developing marker compounds for use in identifying genuine pharmaceuticals often face uncertainty in establishing how to secure regulatory approval for their use.
Companies developing marker compounds for use in identifying genuine pharmaceuticals often face uncertainty in establishing how to secure regulatory approval for their use. 