Germany to join EU medicines verification system
Phil Taylor, 05-Mar-2014
 The European Stakeholder Model (ESM) for verifying the authenticity of prescription drugs will take another leap forward in July when it is linked to a parallel project being set up in Germany.
The European Stakeholder Model (ESM) for verifying the authenticity of prescription drugs will take another leap forward in July when it is linked to a parallel project being set up in Germany.
The German securPharm system will be integrated with the ESM's European hub, testing the hypothesis that national systems will be able to work seamlessly in tandem with the European level architecture of the proposed European medicines verification system (EMVS).
The cloud-based EMVS hub is designed to handle the transactional data generated as unique serialised codes are added to medicine packs at the point of manufacture - and verified at the point of dispensing or (in some cases) by wholesalers.
It is critical therefore that the hub can interface properly with IT systems operated by manufacturers, parallel distributors, wholesalers and pharmacies as well as national database systems if it is to be suitable for pan-European application.
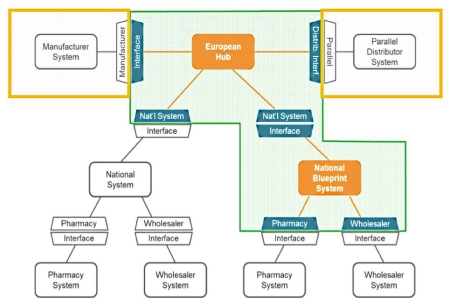
Once the proof-of-principle has been demonstrated in Germany the ESM can then try to replicate that with other national medicine coding systems. Within the EU serialised coding schemes are already operating in countries such as Belgium, Greece and Italy for example.
The ultimate aims is to develop fully integrated supply chain protection across the EU rather than multiple incompatible national systems, said the European Federation of Pharmaceutical Industries & Associations (EFPIA), one of the partners in the ESM.
The requirement for this form of protection is enshrined in the Falsified Medicines Directive (FMD), which became EU law after being transposed into national legislature last year.
The specifics of the medicines verification have yet to be fully determined - that will take place in a delegated act due to be published later this year - although a recent impact assessment from the European Commission suggests the ESM is compatible with its current thinking.
The EC favours a stakeholder based, end-to-end verification system under the supervision of competent authorities and using datamatrix codes.
Commenting on the latest development, Richard Bergström, director general of EFPIA, said: "We look forward to the start-up project as another opportunity to demonstrate the effectiveness of ESMs European hub."
"The collaboration with securPharm is invaluable in demonstrating the hub's potential, furthering our efforts to fight falsified medicines in Europe as a whole [and] I encourage manufacturing authorization holders to join us in moving this initiative ahead," he added.
The alternative model being proposed in Europe is the eTACT system developed by the European Directorate for the Quality of Medicines & Healthcare (EDQM), which places a greater emphasis on tracking medicines at multiple points through the supply chain, allowing more participants to verify the codes (including patients), as well as public sector governance of the system.
The EDQM has been demonstrating its system to carious stakeholders over the last few months, adding point-of-dispense verification functionality last year.

©
SecuringIndustry.com




 The European Stakeholder Model (ESM) for verifying the authenticity of prescription drugs will take another leap forward in July when it is linked to a parallel project being set up in Germany.
The European Stakeholder Model (ESM) for verifying the authenticity of prescription drugs will take another leap forward in July when it is linked to a parallel project being set up in Germany.
