FDA delays UDI requirements for some medical implants
Phil Taylor, 27-Nov-2014
 The US FDA has decided to give labelers of some medical devices more time to comply with its unique device identifier (UDI) rules.
The US FDA has decided to give labelers of some medical devices more time to comply with its unique device identifier (UDI) rules.
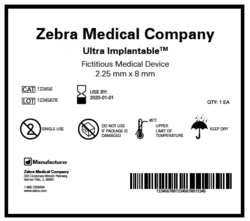
The UDI requirement - which was finalised in September 2013 and became mandatory for high-risk (class III) medical devices in September 2014 - is designed to give each device sold in the market a unique identity that can be used to improve adverse event reporting, allow easier recall of devices, fight against counterfeiting and improve patient safety.
The compliance date for low-risk (class I), moderate-risk (class II) or unclassified implantable devices has been set at September 24, 2015.
Now, the FDA says in a letter that manufacturers of some implantable orthopaedic will have another two years to come into compliance, setting a deadline of 24 September, 2016. The agency has previously granted a one-year extension to manufacturers and labellers of class III contact lenses and intraocular lenses.
The extension has come following a recognition that some implantable devices used in various types of spine, trauma, craniomaxillofacial, or extremity surgeries are cleaned and sterilized prior to use, so are separated from their labels.
Initially, the UDI rule contained a provision requiring implantable devices to be directly marked with a UDI," says the letter. "This proposal was not finalised in the UDI rule because it was presumed that implants would be accompanied by their UDI … up to the point of implantation."
 The extension came about following a petition from device manufacturer AdvaMed, which asked for more time to establish how best to tackle the issue of identifying devices separated from their original label and package.
The extension came about following a petition from device manufacturer AdvaMed, which asked for more time to establish how best to tackle the issue of identifying devices separated from their original label and package.
"The additional time provided by this extension is intended to allow the affected labellers to develop and implement approaches that will help ensure that the UDI is available at the point of use," writes Thomas Gross, director of the office of surveillance and biometrics at the FDA's Centre for Devices and Radiological Health.
The UDI system consists of two core items. The first is a unique number assigned by the device manufacturer to the version or model of a device. This identifier will also include production-specific information such as the product's lot or batch number, expiration date, and manufacturing date if that information appears on the label.
The second component is a publicly searchable database administered by the FDA, called the Global Unique Device Identification Database (GUDID) that will serve as a reference catalogue for every device with an identifier, with no link to patient-level data.
Related articles:

©
SecuringIndustry.com




 The US FDA has decided to give labelers of some medical devices more time to comply with its unique device identifier (UDI) rules.
The US FDA has decided to give labelers of some medical devices more time to comply with its unique device identifier (UDI) rules. The extension came about following a petition from device manufacturer AdvaMed, which asked for more time to establish how best to tackle the issue of identifying devices separated from their original label and package.
The extension came about following a petition from device manufacturer AdvaMed, which asked for more time to establish how best to tackle the issue of identifying devices separated from their original label and package.