FDA finalises guidance for on-dose anti-counterfeit technologies
Phil Taylor, 20-Oct-2011
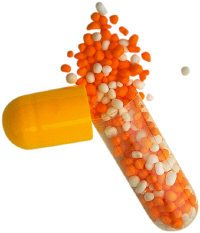 The US Food and Drug Administration has published its final guidance on the use of on-dose anti-counterfeiting technologies - referred to as physicochemical identifiers or PCIDs - for use in solid oral dosage forms such as tablets and capsules.
The US Food and Drug Administration has published its final guidance on the use of on-dose anti-counterfeiting technologies - referred to as physicochemical identifiers or PCIDs - for use in solid oral dosage forms such as tablets and capsules.
The new document extends and finalises earlier draft guidance released in the middle of 2009 (see FDA publishes guidance on anti-counterfeiting markers), and provides advice on the supporting information that drugmakers need to provide in order to secure approval for using PCIDs in their products, either during initial development or as a post-approval change.
As in that earlier document, the agency defines a PCID as "a substance or combination of substances possessing a unique physical or chemical property that unequivocally identifies and authenticates a drug product or dosage form."
The latest version notes that examples of suitable PCIDs include inks, pigments, flavours, and molecular taggants, while stressing that these ingredients should not be pharmacologically active so they can be treated from a regulatory perspective as inactive excipients. The smallest possible amount of a PCID to allow identification and authentication of a product should be employed in order to reduce the risk of side effects.
To that end, the FDA also recommends the use of food additives that have already been cleared under its Generally Recognised as Safe (GRAS) classification. The amount of information provided for a PCID would depend on its pharmacological and toxicological characteristics, as well as the design of the oral dosage form, so use of well-established ingredients will reduce the data requirement.
The guidance also uses the concept of sections - meaning a discrete, contained solid or a layer in a solid oral dosage form - as a means of reducing risk when incorporating a PCID within a drug product.
For example, product developers might consider not incorporating the PCID within the core of a dosage form which contains the active pharmaceutical ingredient (API) in order to minimise the risk of interactions which could result in degradation.
The new document also confirms that PCIDs which stand up to scrutiny will not need to be identified on the labelling unless they change the identifying characteristics of a product, such as its colour.
 Drugmakers have already started to look at incorporating PCIDs into their pharmaceutical products, and last year one technology supplier - NanoInk subsidiary NanoGuardian - said it was within months of its first pharmaceutical customer bringing product to market using its on-dose authentication technology which combines overt, covert and forensic markers.
Drugmakers have already started to look at incorporating PCIDs into their pharmaceutical products, and last year one technology supplier - NanoInk subsidiary NanoGuardian - said it was within months of its first pharmaceutical customer bringing product to market using its on-dose authentication technology which combines overt, covert and forensic markers.
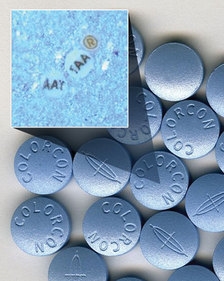
Other key players include excipient specialist Colorcon and its partner ARmark, which last year agreed to work together on an authentication technology that can be incorporated into the film coatings of solid oral dosage forms.
The On-Dose ID technology (pictured) consist of covert micro-tags that can be integrated into existing film coating processes and carry information such as lot and batch numbers, logos and other text, patterns, shapes and symbols, in a particle just 75-110 microns across.

©
SecuringIndustry.com




 The US Food and Drug Administration has published its final guidance on the use of on-dose anti-counterfeiting technologies - referred to as physicochemical identifiers or PCIDs - for use in solid oral dosage forms such as tablets and capsules.
The US Food and Drug Administration has published its final guidance on the use of on-dose anti-counterfeiting technologies - referred to as physicochemical identifiers or PCIDs - for use in solid oral dosage forms such as tablets and capsules. Drugmakers have already started to look at incorporating PCIDs into their pharmaceutical products, and last year one technology supplier - NanoInk subsidiary NanoGuardian - said it was within months of its first pharmaceutical customer bringing product to market using its on-dose authentication technology which combines overt, covert and forensic markers.
Drugmakers have already started to look at incorporating PCIDs into their pharmaceutical products, and last year one technology supplier - NanoInk subsidiary NanoGuardian - said it was within months of its first pharmaceutical customer bringing product to market using its on-dose authentication technology which combines overt, covert and forensic markers.